
Minimize Complexity,
Maximize Versatility
The entire GMK Total Knee Replacement System has been designed to preserve the joint functionality without dramatically altering its anatomy and kinematics, even in cases of severe ligament instability or massive bone defects.
Thanks to the GMK Hinge comprehensive range of options the surgeon can address very unstable knees without compromise, restoring the confidence of movement for his patients.
The stress-free modular mechanism of GMK Hinge implant, together with its bone preserving design, help the surgeon to address demanding joint salvage procedures as well as unstable primary surgeries where the clinical indications show evidence of a constrained implant.
Intuitive instruments help the surgeon to minimise the number of intra-operative steps and be confident that a standardised procedure will make the entire surgery more efficient and time saving.

GMK Hinge is also available with SensiTiN, a ceramic-like coating designed to reduce the release of metal ions from the implant.
The GMK Hinge portfolio includes a comprehensive range of options to address every surgical scenario.
6 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented

Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

6 sizes
Anatomical: left and right
Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4)
Cemented

Material: Cobalt-Chrome (Co-Cr-Mo ISO 5832-4) + SensiTiN coating

Symmetric
Anteriorly flared to accommodate patellar tendon
6 sizes
Seven levels of thickness (10, 12 ,14, 17, 20, 23, 26 mm)
Additional fixation screw and hinge pivot included
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)

Interchangeable femur / tibia
2 offset: 3 , 5 mm
Titanium alloy (Ti6Al4V, ISO 5832-3)

Interchangeable femur / tibia
Diameter = 10,11,12,13,14,15,16,18,20,22 mm
Length = 65, 105, 150 mm
Titanium alloy (Ti6Al4V, ISO 5832-3)

Interchangeable femur / tibia
Tapered shape
Diameter = 11, 13, 16 mm
Length = 65, 105 mm
Titanium alloy (Ti6Al4V, ISO 5832-3)

Mechanically attached to femoral component (screw included)
Interchangeable medial / lateral side
Five levels of thickness: 4, 8, 12, 16, 20 mm
High nitrogen Stainless steel (M30NW, ISO 5832-9) and Titanium alloy screw (Ti6Al4V, ISO 5832-3)

Mechanically attached to femoral component (screw included)
Interchangeable medial / lateral side
Two levels of thickness: 5, 10 mm
High nitrogen Stainless steel (M30NW, ISO 5832-9) and Titanium alloy screw (Ti6Al4V, ISO 5832-3)

Mechanically attached to femoral component (screws included)
Interchangeable medial / lateral side
Four levels of thickness: 5, 10, 15, 20 mm
High nitrogen Stainless steel (M30NW, ISO 5832-9) and Titanium alloy screw (Ti6Al4V, ISO 5832-3)

Anatomical shape
4 sizes
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)
Cemented
Three fixation pegs

Round shape
3 sizes
Machined Ultra High Molecular Weight Polyethylene (UHMWPE ISO 5834-2)
Cemented
One central fixation peg

The femoral component is supplied pre-assembled, saving implantation time.
The hinge mechanism is attached intra-operatively using the standardised torque wrench provided which does not require major luxation of the knee joint and is therefore less invasive of the soft-tissues
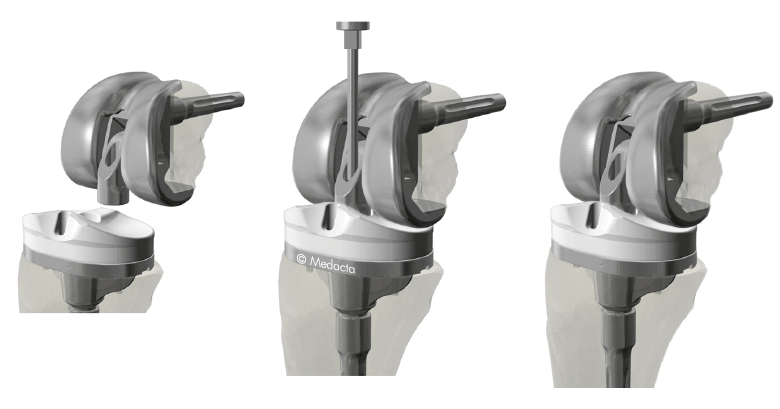

The femoral box dimensions are reduced to a minimum and decrease with the femoral size, saving precious bone stock.
The hinge mechanism does not extend on the posterior condyles thus avoiding substantial bone loss as is not the case with most other implants currently available.

The internal surface of the tibial baseplate is mirror polished, minimising the risk of backside wear. The keel dimensions are the same as for GMK PRIMARY, allowing for the use of a modular offset. Mechanically attached augments are available to fill femoral and tibial bone defects and manage the joint line position.
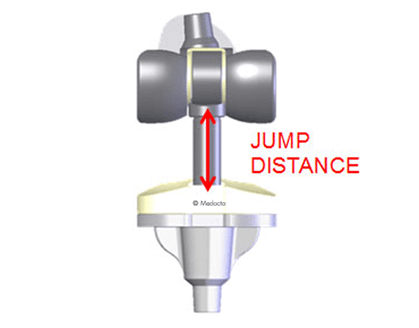
The jump distance, a key indicator of anti-luxation safety, increases with the inlay thickness from 26 mm to 42 mm, increasing exactly where needed, ie. where the knee is more lax and possibily requiring a thicker inlay to be implanted.
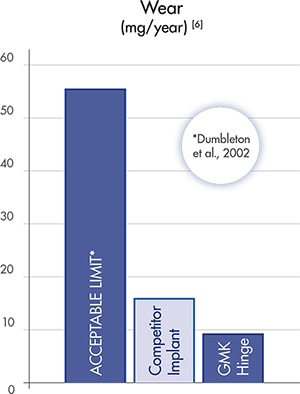
GMK HINGE fixed inlay provides excellent wear resistance, showing significantly lower wear values compared to the acceptable published limit [6].
POLYETHYLENE
Published papers show that polyethylene that does not undergo any irradiation or thermal treatments, that may affect mechanical properties, may show reduced potential of delamination[1]. Medacta provides machined, non-irradiated polyethylene for all GMK tibial inserts.
Interchangeable modular offset adapters are available for the femur and tibia providing maximum flexibility with minimum inventory and avoiding the need for additional offset stems.
All modular connections are accurately tested[6] and dedicated instruments are available in the operating room to ensure reproducible fixation and maximum safety for the patient.
Due to different clinical indications, both cementless and cemented options are available. Cementless stems have longitudinal splines to provide rotational stability, whereas cemented stems have longitudinal pockets for cement and a tapered shape to facilitate insertion.

The primary goal of a GMK Hinge knee replacement includes the restoration of anatomical alignment and functional stability, the accurate re-establishment of the joint line and the fixation and stabilisation of the revision prosthetic implant components.
The GMK Hinge has flexible instrumentation that covers both demanding revision cases and straightforward primary cases, flawlessly adapting to every surgical scenario.
The surgeon can therefore proceed step-by-step with complete confidence even in the most challenging situation.

Dedicated instruments are provided to accurately reproduce the validated offset on femur and tibia.
All modular connections are secured by Morse taper, additional screw, dynamometric wrenches and special impactors which ensure a standardised and reproducible procedure.
The modular hinge mechanism is attached intra-operatively using the torque wrenches provided.

The instruments provided easily allow the surgeon to switch, intra-operatively, from a less constrained to a more constrained inlay when more stability is required.
[1] Ries M D, “Highly Cross-Linked Polyethylene. The Debate is Over-In Opposition”, The Journal of Arthroplasty, 20:59-62, 2005.
[2] Kondo et al. “Arthroscopy for evaluation of polyethylene wear after total knee arthroplasty”, J Orthop Sci, 13:433-437, 2008. Orthop Sci, 13:433-437, 2008
[3] Baker et al., “The effects of degree of Crosslinking on the fatigue crack initiation and propagation resistane of orthopedic-grade polyethylene”, J Biomed Mater Res A, 66(1):146-54, 2002.
[4] Muratoglu et al., “Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE)”, Biomaterials, 20:1463-70, 1999.
[5] Polyethylene in TKA: Do we really need cross-linked polyethylene?, MORE Journal Vol. 1 May 2011
[6] Data on file: Medacta

Same internal femoral profiles of GMK HINGE, GMK REVISION and GMK PRIMARY ensure a full transition through the system, ensuring the freedom to choose intra-operatively the most suitable constraint for each patient, even after the bone cuts have been performed.
In addition, modular add-on instruments trays make the procedure smooth and time-saving, resulting in OR efficiency.

Medacta offers you the opportunity to manage challenging cases, such as uni-compartmental knee revisions or difficult primary cases when augments or intramedullary canal fixation is required. With the enhanced accuracy and proven efficiency of a system specifically designed for each individual patient: this is the synergy between GMK revision system and MyKnee. The accurate pre-operative MyKnee planning combined with the straightforward crossover technique help to easily manage those challenging surgeries in total confidence with a highly reproducible procedure.